
VALORFIN: New Cementitious Materials from the Valorisation of the Fine Fraction of Construction and Demolition Waste
July 22, 2025
QUEEN: Efficient and Sustainable Production of Metallurgical Silicon from Quartz Sand Sourced from Local Quarries
August 26, 202524/07/2025
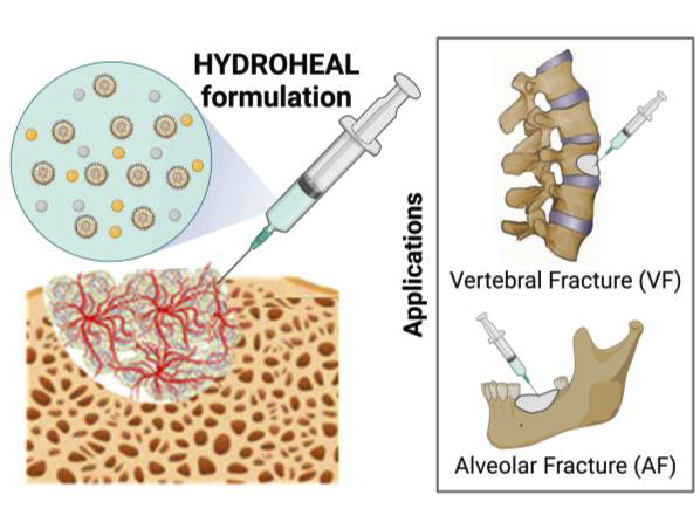
A team from the Bioinspired Oral Biomaterials and Interfaces (BOBI) research group of the Universitat Politècnica de Catalunya - BarcelonaTech (UPC) is taking part in the European project HYDROHEAL, which explores how to transform bone fracture treatment using smart and advanced biomaterials, aiming to reduce the risk of infection and implant rejection, as well as shortening fracture recovery times.
Currently, materials used to replace bone often become infected. To address this, the HYDROHEAL project will specifically evaluate, under conditions that simulate the human body, how bacteria adhere to the developed materials to determine the best strategies for preventing this type of bone infection.
The research team behind the HYDROHEAL project will develop microparticles encapsulated in bioresorbable and self-hardening hydrogels, allowing the incorporation and transport of various pharmacologically active compounds. These biomaterials will serve as temporary support for bone regeneration while also enabling controlled and targeted release of active agents, thereby enhancing treatment effectiveness and significantly reducing the risk of infection and implant rejection. The project focuses on developing new biomaterials for the treatment of vertebral and alveolar fractures (in the jaw, at the base of the teeth), especially in complex cases caused by osteoporosis, cancer or trauma.
The project will employ innovative technologies such as “layer-by-layer” coating to develop the microparticles, adding multiple layers containing active molecules that can be released sequentially during treatment.
HYDROHEAL will also integrate artificial intelligence tools and hybrid digital modelling to optimise the design and performance of the new materials. Following an initial formulation phase, in vitro and in vivo tests will be conducted to validate their efficacy and safety, before moving on to sustainable large-scale production.
Consortium, Budget and Funding
The project, funded by the European Commission’s Horizon programme with a budget of nearly € 6.5 million, will run for a period of four years (June 2025 – May 2029).
Coordinated by UPV, the project brings together 13 partners from 8 European countries, including universities, hospitals, technology centres and companies from the pharmaceutical and biomedical sectors. Alongside UPC and UPV, participating institutions include Newcastle University (UK), Politecnico di Torino (Italy), Royal College of Surgeons in Ireland (Ireland), University of Warwick (UK), Centre for Process Innovation – CPI (UK), SITEC Pharmabio (Spain), Separeco (Italy), Vet Ex Machina LTD (Cyprus), Fluidinova S.A. (Portugal), Asphalion S.A. (Spain), and ConsulTech GmbH (Germany).

Technology
Sector
You want to know more?
Related Projects
- The Image and Video Processing Group (GPI), part of the IDEAI-UPC research group, and the Digital Culture and Creative Technologies Research Group (DiCode) from the Image Processing and Multimedia Technology Center (CITM) at the Universitat Politècnica de Catalunya – BarcelonaTech (UPC), have co-organised the AI and Music Festival (S+T+ARTS) together with Sónar+D and Betevé, to explore the creative use of artificial intelligence in music.
- The Visualisation, Virtual Reality and Graphic Interaction Research Group (ViRVIG) at the Universitat Politècnica de Catalunya - BarcelonaTech (UPC) has participated in the XR4ED project, an initiative that connects the educational technology (EdTech) and Extended Reality (XR) sectors, with the aim of transforming learning and training across Europe.
- The inLab FIB at the UPC has collaborated with Lizcore® for the development of a proof of concept based on artificial intelligence to improve safety in climbing with autobelay devices. The system allows the automatic and accurate detection of risk situations before starting a route.
- Researchers from the Centre for Image and Multimedia Technology of the UPC (CITM) and from the DiCode research group (Digital Culture and Creative Technologies Research Group) of the Universitat Politècnica de Catalunya – BarcelonaTech (UPC) have worked on the project The Eyes of History, an initiative of the Catalan Agency for Cultural Heritage that offers an immersive view of Catalan cultural heritage. It is especially aimed at the first and second cycles of secondary education and was created to bring heritage into the classroom. Its goal is to bring the history and monuments of Catalonia closer in a vivid and innovative way, using tools such as virtual reality and new museographic narratives.




